
Crude Quality Issues
Crude oil is a highly variant natural resource. The quality ranges are similar to coal and depending on the maturation of the crude the quality can be high or low (younger crude's are of lower quality). One of the first indications of quality is color. The variations in oil color can be dramatic, and very indicative of the quality of that crude. Not all crude oil is black - higher quality oils can be a golden or amber in color.
 These crude oil samples are from New Zealand (Source: Trans-Orient Oil)
These crude oil samples are from New Zealand (Source: Trans-Orient Oil)All the quality measures here are based on the ability to produce the desired products. In the U.S., about 50% of the oil is converted into gasoline. So an oil that produces a higher % of gasoline "cuts" is more desirable and have a higher quality oil. Take note, we have used much of the higher quality crude oil already! Now we need to use the lower quality oils too and the general trend is to use increasingly lower quality crude's. This quality reduction has an impact on how we refine the crude into the desirable products.
Viscosity
Viscosity is the resistance to flow. Do not use the term "Thickness" which is a length measurement. The higher the viscosity the slower the liquid will flow and the lower the quality.
 Source: EJS (e-Ed. Institute)The race is on.....which of the crude oils will win this viscosity test?
Source: EJS (e-Ed. Institute)The race is on.....which of the crude oils will win this viscosity test?We have many techniques for measuring viscosity, some of which are quite high-tech. Here is one of the simplest, utilizing one of the testing devices in one of our petroleum labs over in Hosler.
Quicktime
(17 mg)The viscosity process is a measure of quality, because the chemical structure of the crude influences its flow ability. Longer chain molecules for example are harder to flow than short chains because of non-bonding interactions. If you have had any chemistry you will recall ionic (type of bonding in salt crystals) and covalent bonding (the type of bonding between 2 carbon atoms). Those are bonding interactions. There are several non-bonding interactions that occur which attract (and repel) molecules. It is the relative strength of these non-bonding interactions that influences the resistance to flow.
Elemental Composition
For coal we used the correct terminology, which was ultimate analysis. For crude, that terminology we use is Elemental Analysis. Crude oil is complex, it contains C, H, N, S, O, and metals too. But the bulk of the composition is C and H, the rest being the N, S, O and metals. S is a good indication of the quality of the crude because as the oil is heated underground the weak S-C bond can break producing H2S (hydrogen sulfide gas). So, older crudes - higher quality - will have lower S content. Higher S crudes also cost more to process as S is a catalyst poison it has to be removed or the extensive catalysts used in the petrochemical industry would be damaged, as would your catalytic converter. The atomic H/C ratio is also an indicator of quality (why?)Elemental Composition of Typical Crude:
Carbon
84 - 87% Hydrogen
11 - 14% Sulphur
0 - 6% Nitrogen
0 - 1% Oxygen
0 - 2%
Chemical Structures
Hydrocarbons are molecules that contain only the elements of carbon and hydrogen. These are the bulk of the crude oil. We find 4 types of chemical structure of hydrocarbon in crude oil:
Straight-chain Paraffin's Branched-chain paraffin's Aromatics Naphthalenes Paraffin - Straight & Branched
If the CHIME plugin does not work on your computer you can see the movie instead via the links to the example structures (QuickTime format.) We have seen normal (for example n-heptane) and branched (2,2,4 iso-octane) examples of the paraffin's. The all have the same formula: CnH2n+2 (n is the number of carbon atoms). For example, in the cetane molecule to the left, to determine the molecular weight (Mw) you can count the carbons (x 12 the amu of a carbon atom) and count the hydrogen atoms (x 1 amu) and add the numbers together to obtain the molecular weight. Or you can use the formula:
Cetane has 16 carbon atoms (but if we used decane you would know how many carbons it contained, right?) so C16H(2 x 16)+2 OR C16H34 and the Mw is = (12 x 16) + (1 x 34) = 226 amu (atomic mass units).
The paraffins' are the desired contents of the crude oil. Long chains (> 60 carbon atoms are wax) used to be used extensively for the production of candles. Now we use the shorter chains produce gasoline, diesel and jet fuel (and many other products). Note that each molecule might have many structural isomers, for example a molecule containing 10 carbon atoms has 75 structural isomers. If an isomer is an unfamiliar term to you, I'd suggest looking it up using the ANGEL dictionary function above and to the right of your browser window.
Aromatics
Aromatics are found in both crude oil and coal. In crude oil they are now undesirable because of soot production during combustion.
I took this soot picture with a scanning electron microscope so we can see the very small (>1 micron) spherical soot particles. These sphere join together to form chains of spheres. To give you some idea of the scale: 80 microns is about the width of human hair.  This is soot from a diesel engine. Click here or on the image to get a better view
This is soot from a diesel engine. Click here or on the image to get a better viewTake note that the aromatics have a much lower H/C ratio that the paraffin's. The benzene ring contains double bonds (not shown). Aromatics can exist in complex structures containing many rings. The non-bonding interaction between these rings is strong and so pure compounds of 3 rings are solid at room temperature. The equivalent normal paraffin is a viscous liquid under the same conditions.
Naphthenes
Chimes
These are cyclo-paraffins and although this example of cyclohexane looks like a benzene molecule there are no double bonds within the ring and so every carbon (in this example) has 2 hydrogen atoms bonded to it. Cyclohexane has an interesting boat or chair configuration. Can you see the differences?
Classification
In a similar manner to coal as the source rock is buried deeper the temperature increases with increasing depth. Thus looking at quality indicators allows for a classification system similar to that of coal rank.
Because "old deep" oil provides the highest quantity of gasoline, it is the higher quality crude oil.
Most graphs you are used to seeing or plotting have just 2 axes. This works fine if you're just comparing 2 components, but as you see below, we're comparing 3 general classifications for crude oil compound types. It is the ratio of these compound types (aromatics, paraffin's and naphthenes) that impacts the quality of the crude (in addition to S content, especially when the S is within the aromatic portion, which makes it much harder to remove during refining).
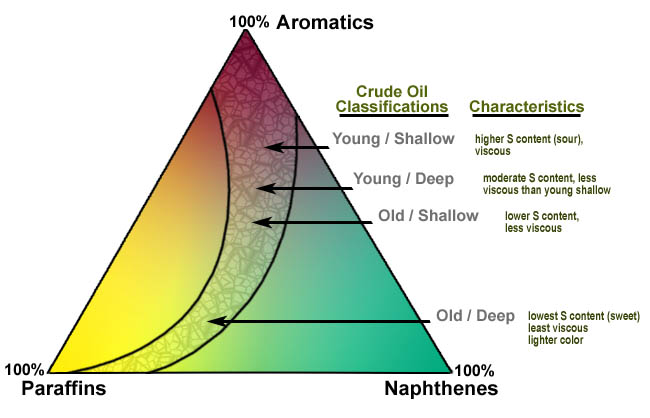 This ternary diagram is used to illustrate the relative percentage of three components in crude oil. The patterned band represents the mixture found in the majority of crude oils.
This ternary diagram is used to illustrate the relative percentage of three components in crude oil. The patterned band represents the mixture found in the majority of crude oils.So, to plot 3 items on a single graph we use ternary diagrams like the one you see above. At the three apexes, the composition would either be pure (100%) aromatics, pure naphthenes or pure paraffins (clockwise from top). Along any of the borderlines of this triangle, you're looking at a mixture of just 2 of these components (aromatics - naphthenes, or naphthenes - paraffins, or paraffins - aromatics). At any point within the triangle, the crude contains all three components, in varying degrees.
Take the example of 50 % aromatics to begin with. To plot this point on the graph, you'd create a drop a horizontal line about half way between the apex (100 %) and the base of the triangle opposite of that apex (0%), representing 50%. You repeat this process to locate the other %'s of the compound types on the graph, and the point you're after is the convergence of those three lines. Thus, the center of the triangle is: 33 %, 33 %, and 33 % of aromatics, naphthalenes and paraffin's, crude oil that would generally fall into the "old shallow" classification. Here is a dynamic exmaple.