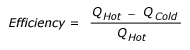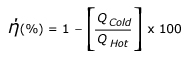| LESSON 3: Energy Efficiency |
||||||||||||||||||
|
3.6 Heat Engines - The Carnot Efficiency |
|
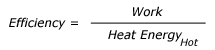
A general expression for the efficiency of a heat engine can be written as: We know that all the energy that is put into the engine has to come out either as work or waste heat. So work is equal to Heat at High temperature minus Heat rejected at Low temperature. Therefore, this expression becomes: Where, QHot = Heat input at high temperature and QCold= Heat rejected at low temperature. The symbol is often (Greek letter eta) used for efficiency this expression can be rewritten as: The above equation is multiplied by 100 to express the efficiency as percent. French Engineer Sadi Carnot showed that the ratio of QHighT to QLowT must be the same as the ratio of temperatures of high temperature heat and the rejected low temperature heat. So this equation, also called Carnot Efficiency, can be simplified as:
Note: Unlike the earlier equations, the positions of Tcold and Thot are reversed. |