What is a diamond?
To put it simply , a diamond is a minera—a nataurally occurring, inorganic solid with a set chemical composition and crystalline structure. Diamonds are composed of one element, carbon. Now you may recall that graphite is also composed of one element, carbon. But what makes these two minerals distinctly different is their internal crystalline structure. Diamond and graphite have two different arrangements and chemical bond types between the carbons. In fact, the chemical bonds in a diamond are so strong that diamond is the hardest mineral on the planet. Only a diamond can scratch the surface of another diamond.
How do diamonds form?
Experiments, and the high density of diamonds, tell us that they crystallize at very high pressures. In nature this means that diamonds are created by geologic processes at great depth within Earth, generally more than 150 kilometers down, in a region beneath the crust known as the mantle. Other processes, explored later in this lesson, bring diamonds to where people can find them.
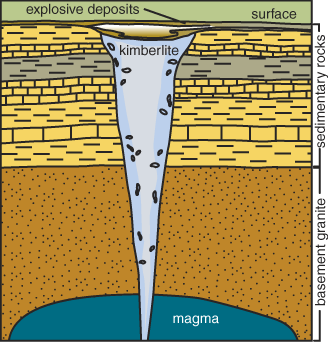
Generalized diagram of a kimberlite pipe. Source: The Geologic Record, Winter 2000 (Kanas Geological Survey).
The Earth is divided into three concentric layers—the core, mantle, and crust. The core is primarily an iron-nickel alloy and makes up a large fraction of the mass of Earth. The mantle is sandwiched between the core and the thin crust and is composed predominantly of magnesium and iron silicate minerals. Our planet's crust is a thin, rocky skin. Diamonds can form in most of Earth's interior but not near its surface. You may be surprised to learn that at surface temperatures and pressures, graphite is the stable form of carbon. In fact, all diamonds at or near the surface of the Earth are currently undergoing a transformation into graphite. This reaction is extremely slow, so there's no danger of any of your jewelry losing value any time soon!
Diamonds ascend to the Earth's surface in rare molten rock, or magma, that originates at great depths. Carrying diamonds and other samples from Earth's mantle, this magma rises and erupts in small but violent volcanoes. Just beneath such volcanoes is a carrot-shaped "pipe" filled with volcanic rock, mantle fragments, and some embedded diamonds. The rock is called kimberlite after the city of Kimberley, South Africa, where the pipes were first discovered in the 1870s. The volcano that carries diamond to the surface emanates from deep cracks and fissures called dikes. It develops its carrot shape near the surface, when gases separate from the magma, perhaps accompanied by the boiling of ground water, and a violent supersonic eruption follows. The volcanic cone formed above the kimberlite pipe is very small in comparison with volcanoes like Mount St. Helens, but the magma originates at depths at least 3 times as great. These deep roots enable kimberlite to tap the source of diamonds. Magmas are the elevators that bring diamonds to Earth's surface.